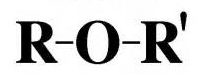Organic Chemistry Classes “OCC”
When you are preparing for pharmacy examination, organic chemistry is very important to understand many biological interactions and drug binding concepts.
I will enumerate different organic classes with important tips about them. Here we go:
Click to enlarge!
Alkanes
- Also called paraffins, saturated hydrocarbons.
- General formula: R-CH2-CH3.
- Lipid soluble.
- Common reactions: halogenation, combustion.
- Chemically inert to air, heat, light, acids, bases.
- Stable in vivo.
Alkenes
- Also called olefins, unsaturated hydrocarbons.
- General formula: R-CH=CH2. Lipid soluble.
- Common reactions: addition of hydrogen or halogen, hydration (to form glycols), oxidation (to form peroxides).
- Volatile alkenes and peroxides may explode in presence of O2 and spark
- Stable in vivo.
- Hydration, peroxidation, reduction may occur.
Aromatic hydrocarbons
- Based on benzene.
- Exhibit multicenter bonding. Lipid soluble. Common reactions: halogenation, alkylation, nitration, sulfonation. Chemically stable.
- In vivo: hydroxylation, diol formation.
Alkyl halides
- Halogenated hydrocarbons. General formula: R-CH2-X.
- Lipid soluble.
- ↑ degree of halogenation↑ Solubility.
- Common reactions: dehyro-halogenation, nucleophilic substitution.
- Stable on the shelf. Not readily metabolized in vivo.
Alcohols 
- Contains OH group.
- May be primary (R-CH2-OH), secondary (R1/R2-CH-OH), or tertiary (R1/R2/R3-C- OH).
- Alcohols are lipid soluble.
- Low molecular weight alcohols are water soluble.
- ↑ hydrocarbon chain length ↓ water solubility.
- Common reactions: oxidation, esterification.
- Stable on shelf.
- In vivo: oxidation, sulfation, glucuronidation.
Oxidation:
primary alcohol aldehydeacid.
Second aryalcoholketone.
Tertiary alcohol not oxidized.
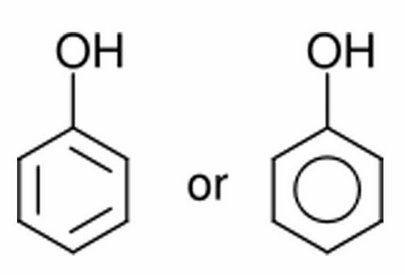
Phenols
- Aromatic compounds containing OH groups directly connected to aromatic ring.
- Monophenols one OH.
- Catecholstwo OH.
- Phenol (carbolic acid): water soluble.
- By ↑ ring substitution ↓ water solubility.
- Most phenols are lipid soluble.
- Common reactions: with strong bases to form phenoxide ion, esterification with acids, oxidation to form colored quinones.
- On the shelf: oxidation with air or ferric ions.
- In vivo: sulfation, glucuronidation, aromatic hydroxylation, o-methylation.
Ethers 
- General formula: R-O-R.
- Lipid soluble.
- Partially water soluble.
- By ↑ hydrocarbon chain ↓ water solubility.
- Common reaction: oxidation to form peroxides (may explode).
- In vivo: o-dealkylation.
- Stability ↑ with size of alkyl group.
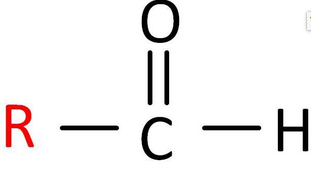
Aldehydes
- General formula: R-CHO (contains a carbonyl group C=O).
- Lipid soluble.
- Low molecular weight aldehytes are also water soluble.
- Common reactions: oxidation (to acids, in vivo and in vitro) and acetal formation.
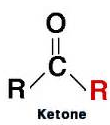
Ketones
- General formula: R-CO-R (contains a carbonyl group C=O).
- Lipid soluble.
- Low molecular weight ketones are also water soluble.
- Non-reactive and very stable on the shelf.
- In vivo: some oxidation or reduction.
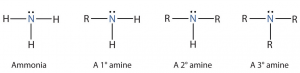
Amines
- Contain an amino group (-NH2).
- Primary (R-NH2), secondary (R1/R2-NH), tertiary (R1/R2/R3-N), quaternary (R1/R2/R3/R4-N+ X-).
- Lipid soluble.
- Low molecular weight amines water solubility.
- By ↑ branching ↓ water solubility (primary amines and most soluble).
- Quaternary amines (ionic) and amine salts are water soluble.
- Common reactions: oxidation (air oxidation on shelf), salt formation with acids.
- Aromatic amines are ↓ basic↓ reactive with acids.
- In vivo: glucuronidatin, sulfation, methylation.
- 1ry: oxidative deaminatin.
- 1y/2ry: acetylation.
- 2ry/3ry: dealkylation.
Carboxylic acids
- General formula: R-COOH (Carboxyl group –COOH).
- Lipid soluble.
- Low molecular weight acid and Na/K salts are water soluble.
- Common reactions: salt formation with bases, esterification, decarboxylation.
- Very stable on shelf.
- In vivo: conjugation (with glucuronic acid, glycine, glutamine), beta oxidation.
Esters
- General formula (R-COOR).
- Lipid soluble.
- Low molecular weight esters are slightly water soluble.
- Common reaction: hydrolysis to form carboxylic acid and alcohol (in vivo by esterases / in vitro).
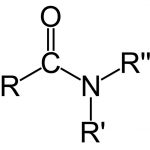
Amides
- General formula: R-CONH2 or R-CONR1/R2 (lactam form).
- Lipid soluble.
- Low molecular weight amides are slightly water soluble.
- No common reactions.
- Very stable on shelf.
- In vivo: enzymatic hydrolysis by amidases in the liver.